On Sanitary Maintenance magazine, may 08 edition, Tennant Co. announced their new ECH2O floor cleaner that electrically activates plain tap water, making it behave like a powerful detergent without any added chemicals. The cleaning effectiveness has no negative environmental impact and health issues associated with producing, packaging and transporting of traditional cleaning chemicals.
sábado, 31 de mayo de 2008
lunes, 26 de mayo de 2008
FISICO QUIMICA DE LAS FORMULACIONES QUIMICAS
La combinacion de diferentes materias primas o ingredientes de diferentes propiedades fisico-quimicas, para formar un producto con propiedades especificas definidas, se denomina la formulacion. La formulacion es el balance meticuloso de todos los ingredientes en una simple entidad fisica, que puede ser por ejemplo un liquido o solido. Usualmente debe existir una "quimica" entre los componentes para poderse obtener las deseadas caracteristicas del producto final; algunos componentes aportan las caracteristicas estructurales, como dureza, estado fisico, viscosidad, etc y otras las caracteristicas funcionalers, como color, lubricidad, solvencia, etc.
En muchos casos las caracteristicas de los componentes cambian su forma y fisico-quimica original, para dar paso a una nueva combinada: por ejemplo cuando se mezcla agua que es un liquido de baja viscosidad, con resina Xantan que es un polvo ligero, se obtiene una solucion de alta viscosidad que es muy homogenea y estable.
En fin, para establecer la correcta formulacion de cualquier producto, primero identifique las caracteristicas y propiedades deseadas para el producto, y luego seleccione las materias primas de acuerdo a sus caracteristicas fisico-quimicas que le puedan proporcionar las deseadas caracteristicas estructurales y funcionales, luego analice si hay "quimica entre ellas, para proceder luego a formularlas.
sábado, 24 de mayo de 2008
NEW PRESERVATIVES FOR TODAY's COSMETICS
Worst news is to realize that your wife's facial cream is not properly preserved and has a foul odor......or that a moisturizer cream has contaminated intensive care patients in a Barcelona's Hospital in Spain. Many new trends for the GREEN drive have put many chemists to work in big Chemical corporations. Traditional preservatives like Formaldehyde donors, Parabens and Isothiazolones have been put aside to give way to natural preservatives, which unfortunately are still not completely defined and not necessarily safe nor effective. Some companies are moving to preservative-free formulations by using actives of a particular class that have known antimicrobial activity like Lactylates or using some ionic surfactants and thickeners that require much less preservatives.
The new trend that seams to be working are the blends that still include Parabens that are believed to be the safest yet in terms of toxicological and use tests. These include products like Clariant's Nipaguard POB, phenoxy ethanol and piroctone olamine and benzoic acid and a second one POM, that combines the two first components with methyl paraben. Lonza's counterpart are Optiphen BSP and BSB which are also aromatics. For a complete report see www.happi.com may2008.
THE CHEMISTRY OF SKIN ACNE CARE
The skin is the largest organ of the human body. One of the most common problems of skin is ACNE, specially for ages between 10 to 15, for both male and females, even though males suffer it more. ACNE is caused by the increase in production of sex hormones (specially the male Androgen), which causes the sebaceous glands in the skin follicles to produce extra sebum that is charged into the hair follicles and eventually clogs the pores, together with the presence of dead skin cells, hair fragments and possibly bacteria.
With this identified, the treatment has to be a combination of preventive measures and the right chemical formulation. The chemical treatment combines a known bacterial killer such as Benzoyl Peroxide ( between 5 and 10%), that oxygenates the environment, combined with surfactants like Linear Alcohols and solvents like Propylene Glycol to dissolve and emulsify the sebum and the thickened vehicle like Xanthan gum to form the surface creamy film. Another Chemical treatment can be using Sulfur that kills bacteria by exfoliating the outer layer of dead skin.
For complete formulation go to http://www.happi.com/ may2008.
In the HAPPI magazine edition of June 2008, there is a report on the creation of cosmetic formulations for remediating Eczema and Dermatitis; the basic ingredients consist of Cetearyl alcohol and sodium cetearyl sulfate 15%, salicylic acid 0.4%, Menthol 0.3% and Water to balance.
This formulation will effectively control itch and pain.
miércoles, 21 de mayo de 2008
NEW GECKO ADHESIVE
It is a great discovery; this new adhesive, made of polypropylene microfibers, developed at the University of California at Berkeley, may be the closest man-made material to match the remarkable gecko toe hairs that allow the tiny lizard to scamper along vertical surfaces and ceilings. Two square centimeters of this material can hold 400 grams (0.88 pounds) weight, vertically bound to a smooth, clean, vertical surface. At the same time, the adhesive easily lifts off with minimal force, leaving no residue.
For a complete report, see Nano News, at www.pcimag.com , May 2008 issue.
martes, 20 de mayo de 2008
NEW CITRIC ACID CLEANER
International Products Corporation Introduces Micro® A07 Citric Acid Cleaner
Citric Acid Cleaner removes dirt without corroding surface.
May 16, 2008 - NSF-Registered for USDA A1 use, Micro® A07 Citric Acid Cleaner removes oxide, scale, mineral deposits, milkstone, and inorganic soils. Biodegradable blend of chelating citric acid and anionic surfactants has typical pH of 2.5 and zero-VOC, phosphate free, non-corrosive formulation. It carries no hazardous shipping regulations and can be used in CIP, ultrasonic bath, immersion, and mild agitation as well as filter membrane applications.
Burlington, New Jersey, USA: International Products Corporation (IPC) announces the launch of their new Micro® A07 Citric Acid Cleaner for removal of oxide, scale, mineral deposits, milkstone, and inorganic soils. This biodegradable blend of chelating citric acid and anionic surfactants offers enhanced cleaning performance compared to simple citric acids and other citric-based products. With a typical pH of 2.5, Micro A07 is milder than most acids, yet powerful enough to replace more aggressive acid cleaners. Micro A07 is used in CIP, ultrasonic bath, immersion, and mild agitation, and filter membrane applications. This cleaner is zero-VOC, phosphate free, non-corrosive, carries no hazardous shipping regulations, and is NSF-Registered for USDA A1 use.
Micro A07 complements IPC's current line of aqueous cleaners, which includes Micro-90® Concentrated Cleaning Solution a powerful alkaline cleaner for use in manual, ultrasonic, CIP, and low-agitation cleaning applications; LF2100® Liquid Low-Foam Cleaner, a high-performance alkaline cleaner for use in high-agitation washers and spray systems; Surface-Cleanse/930® Concentrated Neutral Cleaner, a nonionic, low-sodium detergent for electronic components and delicate metals; Zymit® Low-Foam Enzyme Cleaner, a neutral pH formulation of enzymes and detergents for removal of starches and proteins; and the newly introduced Zymit® Pro Enzyme Cleaner, a neutral pH formula of protease enzymes, detergents, and builders for removal of protein-based soils.
In addition, IPC manufactures and distributes P-80® Temporary Rubber Assembly Lubricants for improved assembly of low tolerance parts. The water-based lubricants provide safe, temporary lubrication during assembly, and then dry to allow a tight fit. P-80 lubricants are widely use in the automotive, appliance, and pump industries, and are compatible with most elastomers, plastics, and metals.
For more information, contact the manufacturer at 609-386-8770 or visit their website: www.ipcol.com. IPC is an ISO-Certified Company.
jueves, 15 de mayo de 2008
PEARLS FOR YOUR HAIR
NEWS FROM NYSCC
Cognis Launches Pearlizing Wax Dispersion
2008-05-14
A novel, mild pearlizer for personal care.
Cognis launched several new materials at the NYSCC Suppliers' Day show in Edison, NJ. For example, the company calls Euperlan Green the first green, ethylene oxide- and amine-free pearlizing wax dispersion. According to Cognis, this novel, tremendously mild and unique pearlizer enables formulators to create natural and attractive hair and body cleansing formulations. It is derived from natural sources, is easy to handle, and is liqud and cold processable, to help save production time and energy costs.Also new is Cosmedia ATH, an anionic liquid dispersion polymer that provides rheology control and facilitates the preparation of skin care emulsions. Supplied as an easily dispersed liquid, it can be used in either cold or hot process manufacturing and can be easily added at any stage of the manufacturing process without pH neutralization. It contributes to the suspension and stabilization of other ingredients because of its excellent thickening performance, according to Cognis.Finally, Cognis rolled out Cosmedia Gel CC, a new version of a hectorite dispersion with unique properties. It creates a homogeneous film on the skin and limits the sedimentation process of pigments in a final formulation—making it an excellent product for sun care, AP/Deo and color applications, according to the company.More info: www.cognis.com
POLYMERS
Polymers, by definition are entities of many parts. In chemistry they constitute the elemental molecule in most materials in the human body and the environment. Proteins, lipids, sugars, tissues are some examples of polymers in our body; in the environment, we can mention wood, wax, starch, crab and turtle covers, oils and greases and so on. Artificially or synthetically, we can produce near any kind of type and structured materials, starting with the appropriate "monomers" that are "polymerized" or linked together to a predetermined length to obtain the desired qualities and properties. Some examples of these are the plastic bags obtained from Polyethylene, a plastic bottle obtained from Polypropylene or a textile fibre obtained from a Polyester or a Polyamide like Nylon. Other examples are polyacrylates and polyurethanes used in floor care finishes and waxes or varnishes, adhesives and paints. For a general definition on polymers and more applications, go to www.adhesivesmag.com and look in the May 2008 issue.
martes, 13 de mayo de 2008
Peristaltic or Diaphragm Metering Pump ??


There can be as many definable parameters in a specific system as there are required functions. Although it would be impossible to identify all of them, the basic metering pump system parameters are: fluid, pressure, control capability and required maintenance. We can analyze each of these parameters with respect to a diaphragm and peristaltic metering pump system and compare their effect on the reliability of the overall system.
Suction strainer with check valve – typically includes a filter screen, valve body, check valve/ball, rubber seal/seat and a metal spring.
Suction tubing or piping.
Pump head with inlet/outlet valves – includes a pump head, valve bodies, check valve/balls, rubber seal/seats and (depending on the manufacturer) metal springs.
Discharge tubing or piping.
Injection fitting with check valve – typically includes a valve body, check valve/ball, rubber seal/seat and a metal spring.
Peristaltic pumps employ much fewer “wetted” components:
Suction tubing or piping.
Peristaltic pumping tube – typically includes inlet and outlet fittings.
Discharge tubing or piping.
Injection fitting with check valve – typically includes a valve body, check valve/ball, rubber seal/seat and a metal spring.
Both diaphragm and peristaltic metering pumps have proven themselves in a multitude of commercial, industrial and municipal chemical metering applications. Still, each pump type has its strengths and weaknesses. A quick review of the system parameter requirements can guide the user in selecting the best pump type for the specific application.
About the Author: Bill McDowell is a sales engineer with Blue-White Industries, of Huntington Beach, CA. With nearly 25 years at Blue-White Industries, he also has held positions as project engineer and engineering director. Contact: 714-893-8529, sales@blue-white.com or http://www.blue-white.com/
GREEN PACKAGING FOR SUSTAINABILITY COMPLIANCE
There's no doubt that a cultural shift is underway, a move towards saving the planet for future generations, and everyone is now striving to balance social environmental and economical concerns. While most are far from reaching a perfectly sustainable world, many companies and governmental institutions are making efforts in this directions. Sample of this is the replacement of plastic bags for paper bags in many cities and the use of materials such as hemp, recycled car tyres and organic cotton in the manufacture of shoes and purses. In the chemical world a lot of interest and effort is being placed towards the actual chemical ingredients in a formula, however the packaging is as important if not more since they are mostly plastics which have long environmental life. Now days many plastics, specially thermosetting are being substituted by aluminum or other more recyclable materials. Also many packaging are being produced from vegetable raw materials like the case of the developments from Nature Works that work from corn stock. For more details go to: http://www.natureworksllc.com/ . Also some companies like Wall Mart are requesting suppliers to meet criteria based on sustainability and recyclable parameters. For more information on green packaging see: http://www.beautypackaging.com/, April/May 2008 issue.
On this same line of events, the DuPont awards for packaging innovation in 2008 went GREEN, awarding a number of notable achievements in sustainable packaging. One of the distinguishable achievements was awarded to the "powerhouse" produce package of Kiwi fruit. The natural package is assembled with a vacuum-thermoformed tray made of fibers from a palm tree from Earthcycle Packaging that is overwrapped in a transparent film of regenerated cellulose from Innovia Films. To see more go to the May edition of www.packworld.com .
MRSA INFECTIONS AT SCHOOLS
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) infection is caused by Staphylococcus aureus bacteria — often called "staph." Decades ago, a strain of staph emerged in hospitals that was resistant to the broad-spectrum antibiotics commonly used to treat it. Dubbed methicillin-resistant Staphylococcus aureus (MRSA), it was one of the first germs to outwit all but the most powerful drugs. MRSA infection can be fatal.
Staph bacteria are normally found on the skin or in the nose of about one-third of the population. If you have staph on your skin or in your nose but aren't sick, you are said to be "colonized" but not infected with MRSA. Healthy people can be colonized with MRSA and have no ill effects. However, they can pass the germ to others.
Staph bacteria are generally harmless unless they enter the body through a cut or other wound, and even then they often cause only minor skin problems in healthy people. But in older adults and people who are ill or have weakened immune systems, ordinary staph infections can cause serious illness.
In the 1990s, a type of MRSA began showing up in the wider community. Today, that form of staph, known as community-associated MRSA, or CA-MRSA, is responsible for many serious skin and soft tissue infections and for a serious form of pneumonia. For more information go to:http://www.mayoclinic.com/health/mrsa/DS00735.
What type of infections does MRSA cause?
• In the community most MRSA infections are skin infections that may appear as pustules or boils which often are red, swollen, painful, or have pus or other drainage. These skin infections commonly occur at sites of visible skin trauma, such as cuts and abrasions, and areas of the body covered by hair (e.g., back of neck, groin, buttock, armpit, beard area of men).
• Almost all MRSA skin infections can be effectively treated by drainage of pus with or without antibiotics. More serious infections, such as pneumonia, bloodstream infections, or bone infections, are very rare in healthy people who get MRSA skin infections.
How is MRSA transmitted?
• MRSA is usually transmitted by direct skin-to-skin contact or contact with shared items or surfaces that have come into contact with someone else's infection (e.g., towels, used bandages).
What type of infections does MRSA cause?
• In the community most MRSA infections are skin infections that may appear as pustules or boils which often are red, swollen, painful, or have pus or other drainage. These skin infections commonly occur at sites of visible skin trauma, such as cuts and abrasions, and areas of the body covered by hair (e.g., back of neck, groin, buttock, armpit, beard area of men).
• Almost all MRSA skin infections can be effectively treated by drainage of pus with or without antibiotics. More serious infections, such as pneumonia, bloodstream infections, or bone infections, are very rare in healthy people who get MRSA skin infections.
Should schools close because of an MRSA infection?
• The decision to close a school for any communicable disease should be made by school officials in consultation with local and/or state public health officials. However, in most cases, it is not necessary to close schools because of an MRSA infection in a student. It is important to note that MRSA transmission can be prevented by simple measures such as hand hygiene and covering infections.
Should the school be closed to be cleaned or disinfected when an MRSA infection occurs?
• Covering infections will greatly reduce the risks of surfaces becoming contaminated with MRSA. In general it is not necessary to close schools to "disinfect" them when MRSA infections occur. MRSA skin infections are transmitted primarily by skin-to-skin contact and contact with surfaces that have come into contact with someone else's infection.
• When MRSA skin infections occur, cleaning and disinfection should be performed on surfaces that are likely to contact uncovered or poorly covered infections.
• Cleaning surfaces with detergent-based cleaners or Environmental Protection Agency (EPA)-registered disinfectants is effective at removing MRSA from the environment.
• It is important to read the instruction labels on all cleaners to make sure they are used safely and appropriately.
• Environmental cleaners and disinfectants should not be used to treat infections.
• The EPA provides a list of EPA-registered products effective against MRSA: http://epa.gov/oppad001/chemregindex.htm
lunes, 12 de mayo de 2008
WHAT IS THE HEALTH SITUATION FOR THE USE OF CHLORINATED SOLVENTS
Late toxicological studies and occupational hazard studies on Chlorinated solvents have placed most organic chlorinated hydrocarbons in the list of carcinogens. These chemicals are normally used in applications like:
- Dry cleaning operations
Fluorocarbon manufacture
Solvents for fats, oils, waxes, resins
Fire extinguishers
Organic synthesis
Polymer manufacture
Heat exchange medium
Chemical intermediate
Extraction of caffeine - Vapor degreasing
Parts cleaning
Engine cleaner
Degreasing agent
Cleaning printed circuit boards
Adhesives solvent
Aerosol propellant
Foam plastic blowing agents
Paint removers
Breathing small amounts of these solvents can cause headaches, lung irritation, dizziness, poor coordination and difficulty concentrating. Breathing or drinking large quantities of these solvents can cause impaired heart function, unconsciousness, long term kidney and liver damage and possibly death.
Please see the link http://www.meridianeng.com/chlorina.html for a complete list of properties and health facts for the most common chlorinated solvents.
Replacement for Hazardous or Chlorinated Solvents
Metalworking plants, metal service centers, fabricating, finishing facilities and rebuild shops should consider using PICO SOLV NPB to eliminate problems such as: residues in trapped areas, solvent fire hazards, rusting caused by water based cleaners, slow drying times and odor complaints. Typical applications include: oil removal from screw machined parts, dirty electrical components, oxygen service cleaning and coil-to-coil metal cleaning. PICO SOLV NPB is compatible with most metals, plastics and space-age materials and is inhibited against white metal staining and corrosion. If there is doubt about any material, users are encouraged to contact PICO's technical personnel.
PICO SOLV NPB does not contain petroleum solvents, chlorinated compounds, or water for improved health, safety and environmental compliance. PICO SOLV NPB is easily used in vapor degreasers, ultrasonic agitators, dip tanks, enclosed spraying systems or wiping. It is a stabilized azeotropic mixture that may be distilled many times and has good wetting characteristics to evenly coat surfaces, thus releasing its full power solvency to penetrate and dissolve oil and grease films.
jueves, 8 de mayo de 2008
CERA / WAX FOR POLISHING BOATS AND BOARDS


SHOULD YOU USE CHLORINE IN LIQUID, SOLID OR GAS FORM, WHEN TREATING WATER ??
Application Bulletin
Bulletin 3001
Cost Comparison
Chlorine Gas vs. Hypochlorite
Cost per pound of AVAILABLE CHLORINE:
Gas Chlorine - is 100% pure, elemental chlorine which maintains its strength indefinitely.
Average cost per pound is $0.45‡. Since it is pure chlorine, the cost per pound of AVAILABLE CHLORINE = $0.45/lb.
Powder Chlorine (Calcium Hypochlorite) - contains a maximum of 70% available chlorine.
Strength is rapidly lost in storage. Average cost per pound of powder chlorine is $1.23‡. Therefore, the cost per
pound of AVAILABLE CHLORINE = $1.76/lb.
Liquid Bleach (Sodium Hypochlorite) - contains approximately 10% † available chlorine.
Strength is rapidly lost in storage. Average cost per gallon is $1.29‡. One gallon weighs approximately 9 lbs. The
cost of 1 lb. of bleach is calculated at $1.29 / 9 = $0.143. At 10% strength the average cost per pound of
AVAILABLE CHLORINE is determined as $0.143 X 10 lbs. bleach = $1.43/lb
‡ Costs are average quotes from suppliers in Ft. Pierce, Florida metropolitan area, Nov. 1993. Costs may vary in
your area, but the relative differences in costs of the three types of chlorine tend to remain consistant.
† (Note: Most manufacturers of Sodium Hypochlorite will only guarantee a 10% chlorine strength in 16% solution,
due to loss of strength in shipment and storage.)
A study was carried on the use of these three alternatives on a base usage from 1 Lb to 100 Lb each day, between 100 and 350 days. The results for all of them showed that liquid bleach is the most expensive alternative, followed by the powder and having the gas as the much lower cost between them. However the use and dosage of gas can be very delicate and dangerous.
USE OF SILVER IN DISINFECTION



* New disinfectant ingredient silver ions: The new disinfectant silver ion ingredient featured in the figure above, is blended using Lion's proprietary technology which causes the mineral substance alumina silica to retain microparticulated silver ions on the surface. Just 15 nanometers in diameter or about one thousandth the size of typical silver particles, their microscopic size boosts disinfectant effectiveness, allowing them to fully penetrate and defend surfaces from bacteria.
martes, 6 de mayo de 2008
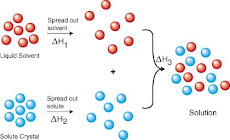
BASIC DEFINITIONS ON HOW TO MAKE EMULSIONS
When two liquids are immiscible but do not separate immediately they are said to form an emulsion. Some emulsions are quite stable and will take a long time to separate. For example milk is an emulsion of water and fat but is fairly stable. Other emulsions may separate quite quickly, for example a simple salad dressing of oil and vinegar will separate almost immediately (note that vinegar is water based).
The emulsion itself consists of small droplets of one liquid within the body of a second liquid. An emulsion containing droplets of oil in water is called an oil-in-water emulsion and the oil is called the dispersed phase while the water is called the continuous phase. An emulsion with droplets of water in oil is called a water-in-oil emulsion. A good oil-in-water emulsion will consist of very fine oil droplets homogeneously dispersed throughout the body of water.
Since colas are oil-in-water emulsions this discussion of emulsions assumes that an oil phase is being dispersed within a water phase. In practice neither the water phase nor the oil phase of an emulsion are likely to be pure substances. For example in colas the continuous water phase is an acidic solution of citric or phosphoric acid together with other ingredients such as caramel and sugar, the oil phase is a complex mixture of organic molecules from the essential oil flavourings. In general the water soluble molecules all stay in the water phase and the oil phase will be a mixture of all the liquid molecules that are not soluble in water (i.e. a mixture of oils).
FOR COMPLETE ARTICLE GO TO: http://sparror.cubecinema.com/cube/cola/chemistry/cola1.htm#top
lunes, 5 de mayo de 2008
MARBLE CARE AND CLEANING
The best way to remove stains on your natural stones surfaces, is to avoid them.
As a spill occurs, blot the spill with a paper towel immediately; do not wipe the area since it will spread the spill and potential stain. Flush the area with plain water and mild soap and rinse several times to completely remove spill. Dry the area thoroughly with a soft cloth. If the stain remains, then refer below to the procedure for stain removal that corresponds.
Stain Removal
The key to remove a stain from marble or stone, is to identify the type of stain. Investigate first as what caused the stain, where is it located, shape and color. Surface stain can often be removed by appropriate household cleaners; deeper stains that are absorbed or have penetrated the stone porous surface are more difficult to remove and may require an absorbent-solvent compound or a professional grade cleaner.
Cleaning Based on Stain Type
Oil or solvent based stains like cooking oil, motor oil, milky drinks, cosmetics,grease, tar and some inks, will darken the stone and normally must be dissolved so that the source of the stain can be flushed or rinsed away. This can be done by using one of the following: alcohol, mineral spirit, acetone or a butyl based degreaser. Simply apply on stain, allow to dissolve stain and clean gently with a soft cloth. Absorb as much as possible then rinse with a neutral cleaner and water.
Organic based stains like coffee, tea, fruit, tobacco, food, urine, leaves, bark, bird droppings all cause a pinkish-brown stain that in many cases will disappear if the source of the stain is removed as soon as it occurs; outdoors, with the source quickly removed, normal sun and rain action will generally bleach out the stains. In other cases and indoors, clean with a 12% Hydrogen Peroxide (hair bleaching strength) and a few drops of ammonia. Handle all chemicals with due care.
Metal oxides like iron oxides, rust and corrosion stains are orange to brown in color and follow the shape of the staining object such as nails, bolts and nuts, cans, metal furniture. Copper and bronze objects leave stains that are dark green-amber. These stains are very difficult to remove since they get embedded and adsorbed inside the stone's veins. The best way to remove them is using a paste (called poultice), made with an absorbent powder like kaolin or silica gel and a mild acid to clean the oxide. The poultice is spread on the stained surface with a spatula, covered with a plastic film and allowed to absorb the stain for at least 12-24 hours. This operation can be repeated if needed; however some deep stains may never be completely removed and sanding and buffing with steel wool may be necessary, followed by polishing or crystallization.
Biological or Organic stains, like mildew, mold, moss, algae, musk or fermented food residues can be removed using a solution of 1 cup of chlorine bleach or hydrogen peroxide in a gallon of water. You could also use the same dilution with an ammonia cleaner but this may have an unpleasant odor and is very harmful if it comes in contact with bleach.
Ink or Paint. Water based stains will be easily removed with a water based degreaser or a neutral cleaner. Oil based stains have to be removed with a solvent based paint stripper, acetone or lacquer thinner. Also use a wood or plastic scraper if necessary to remove stains. NEVER USE CAUSTIC PRODUCTS OR STRONG ACIDS TO REMOVE STAINS since they will damage or opaque the gloss of the stone and will require repolishing or recrystallization.
domingo, 4 de mayo de 2008
QUATERNARIES VS PHENOLICS AS DISINFECTANTS
QUATERNARIES
- Readily combine with non-ionic surfactants, making excellent cleaners since they are great detergents them self.
- They are water soluble.
- Work best at pH round 10, which is best for soap detergency.
- They provide high foam but also fast rinsing.
- Relatively safe to use, some are approved for use as topical and as no rinse sanitizers in food handling areas.
PHENOLICS
- Are inactivated by non-ionic surfactants, must be combined with anionic surfactants to formulate cleaners; these have lower cleaning power than non ionics .
- Do not solubilize in water, detergents are needed to suspend them in water, or alcohols to solubilize them.
- Maximal biocidal activity is achieved at pH of 6 to 7, when detergency is poor.
- Poor rinsing properties and residues left by anionic soaps makes inappropriate for floor cleaning.
- They have questioned safety for skin contact.
- They have greater residuality than quaternaries.
NATURAL BASED INGREDIENTS FOR PERSONAL CARE PRODUCTS
Organic, Green, Natural, all characteristics we would like to have for the chemical industry. Well, at least cosmetics and personal care products are closer. One example is the new line of Polyamides, which are family related to proteins in chemical structures, that are offered as thickeners in the formulations of Mascara, Sunscreen lotions, Anti-aging Lotions and Lipsticks. These low molecular weight Polymers are nature-based, skin-friendly, easy to incorporate can turn almost any liquid into a clear soft solid and allow the formation of emulsions with little or no surfactants. Actually some are lipophilic and therefore water repellent while some are hydrophilic and therefore water dispersible or water soluble. They provide enhanced gloss,a unique skin feel, insensitive to pH and electrically neutral. In general they are composed of polymeric chains formed by reaction of diamines with long chain di-acids, that can form tridimensional structures through Hydrogen bonding when in solution.
For more information see www.happi.com magazine, February 08 issue.
Vistas de página en total
GREEN CHEMICALS
Also the materials have to meet with toxicity and health requirements regarding inhalation, dermal and eye contact. There is also a specific list of materials that are prohibited or restricted from formulations, like ozone-depleting compounds and alkylphenol ethoxylates amongst others. Please go to http://www.greenseal.com/ for complete information on their requirements.
For information on current issues regarding green chemicals, see the blog from the Journalist Doris De Guzman, in the ICIS at: http://www.icis.com/blogs/green-chemicals/.
Certification is an important — and confusing — aspect of green cleaning. Third-party certification is available for products that meet standards set by Green Seal, EcoLogo, Energy Star, the Carpet & Rug Institute and others.
Manufacturers can also hire independent labs to determine whether a product is environmentally preferable and then place the manufacturer’s own eco-logo on the product; this is called self-certification. Finally, some manufacturers label a product with words like “sustainable,” “green,” or “earth friendly” without any third-party verification.
“The fact that there is not a single authoritative standard to go by adds to the confusion,” says Steven L. Mack M.Ed., director of buildings and grounds service for Ohio University, Athens, Ohio.
In www.happi.com of June 2008 edition, there is a report of Natural formulating markets that also emphasises the fact that registration of "green formulas" is very confused at present, due to lack of direction and unification of criteria and that some governmental instittion (in my opinion the EPA) should take part in this very important issue.