The accumulation of soil on textiles is one of the factors that cause textiles to deteriorate.
Spilled food, for example, can turn a textile that is normally unappetizing to insects into an attractive meal for moths and carpet beetles. The "ground-in" dirt can increase the abrasion of yarns, causing them to weaken and lose luster.
Soil removal is one of the most important aspects of caring for textiles if they are to be maintained in good condition.
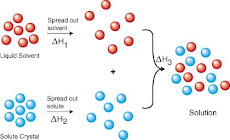
Soil deposited on textiles is made up of different materials. Some types of soil are soluble in water, other types are insoluble. Soluble soil is made up of organic acids, mineral acids, alkaline substances, blood, starches and sugars. All of these substances dissolve in cool or warm water, although they may require special stain removal techniques.
Water is an effective solvent used widely in the process known as wet cleaning.
Water alone will remove water-soluble soil, but insoluble soils may be held onto the textile by physical means such as films, greases or oils. Such soils require the use of some kind of cleaning aid.
For any detergent to clean, it has to interface with the soil. Unlike laundering, in carpet cleaning, whether shampooing or hot water extraction, the detergent comes in contact with the soil for a very short period of time.
In shampooing, very little water is employed and in hot water extraction there is less mechanical action to dislodge the soil particles. Many professional cleaners apply presprays to heavily soiled areas.
Just as dried-out food left on a plate is much easier to clean if pre-soaked, research has shown that leaving presprays on the soiled areas for about 10 minutes results in significant improvement in performance, and will greatly aid in spot removal (stains being more challenging and needing additional treatment).
If the prespray is left on for too short a time period, the improvement will be too small; if left for too long a time period, there will be a risk of overwetting the carpet, causing distortion, browning, dye bleed or rapid re-soiling after cleaning.
You have to keep in mind you are cleaning the carpet surface and not the backing.
Using organic solvents
Organic solvents, which are often called "dry solvents," can dissolve some types of dirt; agitation is often necessary to aid dirt removal. Sometimes surfactants and water are added to the organic solvent.
The low surface tension of all dry solvents allows wetting of the textile fibers without the addition of surfactants.
Rinsing and drying completes the solvent cleaning process.
However, wetting of fibers is not a sufficient prerequisite for satisfactory cleaning results. Wetting allows close solvent fiber interaction.
In hydrophilic textiles like wool, cotton and rayon, swelling is not a big issue, but in non-polar fibers (see "Polarity" sidebar) such as polyester or olefin, the dry solvent could pose a problem by retaining the dry solvent in the textile and affecting its properties.
Normally, a solvent forms a uniform solution with the soil it dissolves. Most commercial cleaners use solvents that evaporate rapidly so that the bulk of the solvent can be removed by good ventilation.
A good, effective dry solvent will dissolve the soil rapidly, evaporates quickly, removes stubborn soils like waxes or polymers, is non-flammable with reduced toxicity; but unfortunately, with ever accumulating new environmental/health regulations, it has become impossible to find an ideal solvent fitting all the criteria.
Troublesome stains
There are times in the process of carpet cleaning, such as shampooing or hot water extraction, that you encounter stains that will not respond to normal cleaning procedures.
In these cases, there are a variety of materials and processes that can help remove these stains. They can range from solvents, oxidizing/reducing agents, freezing agents for removing chewing gum, gels, rust removers, acids, alkalis, etc. How long should the contact time be? It all depends on the stain and procedure attempted.
If the stain is fresh, it may not present much of a problem; if it is urine, a spray extraction machine will generally suffice.
But if it is a colored fruit drink on a nylon 6 carpet, the color uptake by the carpet is rapid; with nylon 6,6, it is slower but still occurs. You may be able to lessen the intensity of the stain by utilizing three percent hydrogen peroxide and using the heat transfer method described in the next paragraph.
Use a 50/50 mixture of hydrogen peroxide and sudsy ammonia, dampen a white cotton towel and apply it onto the stain, then place a hot electric iron (past the steam setting) on the wet cotton towel and you will note some transfer in color. Repeat the procedure using another area on the towel, (use impermeable rubber gloves as the hydrogen peroxide evaporates slower than water and gets concentrated and is liable to burn your skin). By this method, the carpet dye may transfer as well, so be careful.
Bleaching a stain using an oxidizing or a reducing agent will show rapid results, while the use of enzymes to digest a stain will take longer.
Solid stains also take longer; if the material can be broken up by a spatula or a stainless steel spoon, it will increase the surface to help break it down.
If dealing with wax, a warm iron can be used over the absorbent paper towel, but do not overheat and melt the fibers. Chewing gum will require freezing and/or solvent.
Be careful with organic solvents as they may be flammable, toxic or may adversely affect the backing seams. Sometimes, marking pens are used on the inside of upholstery furniture and they may bleed through. When using solvents, use the mildest solvent first, such as non-polar mineral spirits, and gradually move towards more aggressive solvents, such as glycol ethers, and rinse.
Nasty surprises
There are some stains that may only appear after the carpet has been cleaned.
These could be latent stains due to previous sprays, spills or by tracking (intended or unintended) products such as insecticides, foot powder, bleaches or face creams, which can destroy carpet dye.
There are many residues that, upon becoming wet due to carpet cleaning moisture, cause carpet dyes to fade or to be removed completely.
If this happens to you, you can try to explain to the customer that the cleaning solution used to clean the carpet, coming in contact with the affected area of some unknown residue, has adversely affected the area. This is not something the customer will want to hear, but it is accurate information.
Light or fume faded stains are not really stains, but discolorations, and will not respond to cleaning. In such cases, it may become necessary to spot dye the carpet or replace the section from a remnant, if one is available, or from an inconspicuous area, such as a closet. The color of the replaced piece may appear brighter than the rest of carpet, and the wear pattern may not match as well
viernes, 9 de marzo de 2012
THE CLEANING OF FABRICS AND CARPETS, Some Concepts and Tips
jueves, 8 de marzo de 2012
CELLULOSE THICKENERS
Take a Closer Look At Cellulose Thickeners
Some of the most useful thickeners for aqueous systems are cellulose derivatives. They also furnish other qualities. In the construction industry, they control the water binding ability of cement, gypsum and fillers. They perform the same function in wallpaper paste. As additives in laundry detergents, they prevent graying and discoloration. As thickeners in the food industry, they enhance composition, form, structure and consistency. In tablets in pharmaceuticals, they are binding agents and help release the active ingredients. Of course, cellulose derivatives are widely used as thickeners in the cosmetic industry.
The chart below shows how some cellulose ether derivatives are created and their water solubility.
In cosmetics, cellulose derivatives can be used in both shampoos and conditioners. In shampoos, besides providing viscosity, they boost and stabilize foam and add a creamy texture to formulations. They are stable in salt and cationic solutions, increase viscosity across a broad pH range and allow the use of little or no salt. In conditioning systems, they become viscous in the aqueous phase through hydrogen bonding. The products are pseudoplastic, spread easily and rinse off easily from the hair. Cellulose derivatives can also help to stabilize emulsions.
AkzoNobel, Bridgewater, NJ, manufactures the cellulose derivatives listed below. The number after the name denotes the typical viscosity of a 1% solution in water.
*Structure Cel 12000 M
Methyl Hydroxyethyl Cellulose
Structure Cel 4400 E
Ethyl Hydroxyethyl Cellulose
Structure Cel 500 HM
C12-16 Alkyl PEG-2 Hydroxypropyl
Hydroxyethyl Ethylcellulose
*This is also available in a lower viscosity 8000 M grade.
An example of its use in hair products follows:
Sulfate-Free Shampoo
Ingredients:
%WT.
Water
83.0
Sodium C14-16 olefin sulfonate
7.5
Cocamidopropyl betaine (and) water
7.5
Structure Cel 8000 M
1.5
DMDM hydantoin (and) iodopropynyl butylcarbamate
0.5
Procedure: Stir constantly. Mix first three ingredients in tank. Slowly sift in Structure Cel and heat to 40°C. When hydrated, cool to room temperature and add last ingredient. This yellow product is clear to slightly hazy, has a pH of 6-7, and a viscosity of 18,000 to 22,000 cps.
Harvey M. Fishman
Consultant
Harvey Fishman has a consulting firm located at 34 Chicasaw Drive, Oakland, NJ 07436, hrfishman@msn.com, specializing in cosmetic formulations and new product ideas, offering tested finished products. He has more than 30 years of experience and has been director of research at Bonat, Nestlé LeMur and Turner Hall. He welcomes descriptive literature from suppliers and bench chemists.
TAKEN FROM HAPPI, GLEAMS & NOTIONS, JAN 2012
LONZA GROWTH IN DISINFECTANTS
A Winning Combination
Lonza’s $1.2 billion acquisition of Arch Chemicals creates a global leader in the $10 billion microbial control segment.
Tom Branna • Editorial Director
It’s the perfect fit of complementary chemistry and hard assets, according to company executives. The completion of Lonza’s $1.2 billion acquisition of Arch Chemicals last year puts a broad range of microbial control solutions under one roof for the personal care, household, industrial and institutional cleaning markets.
The acquisition of Arch, which reported sales of $1.4 billion in 2010, also made Lonza the leader within the $10 billion global microbial control market, which is growing 4-5% a year, according to industry observers.
But while the sheer size of the acquisition is impressive, a Lonza executive is quick to point out that the big winners to emerge from the deal will be customers.
“Our customers will benefit from a broader portfolio of registered active ingredients and formulations,” explained Frank Kicklighter, Lonza Microbial Control’s head of marketing and communications. “We have enhanced capabilities in toxicology, regulatory and innovation and we can now offer these services to a broader range of customers in both emerging and established markets.”
Lonza has a long history as a leader in cosmetic preservatives including hydantoin and isothiazolone chemistry. Some of the preservatives in the Lonza lineup include Dantogard 2000, a high performance preservative for household and industrial applications that’s based on DMDM hydantoin. The EPA-registered, cost-effective preservative provides broad-spectrum activity and is typically used at 0.05-0.4%.
What Arch Brings
For its part, the Arch business is the world’s leading supplier of zinc pyrithione, the No. 1 anti-dandruff ingredient, as well as natural and organic cosmetic ingredients, cosmetic preservatives and biotechnological actives.
In addition, it offers expanded technologies for cleaning, disinfecting, sanitization and preservation in many business segments, including household/consumer, institutional, industrial, food safety and healthcare.
In terms of geographic reach, Arch brings facilities in North America and Europe, as well as in South Africa and across the Asia-Pacific region.
More, More, More
At press time, Lonza was still in the integration phase regarding its Arch purchase, but Kicklighter assured Happi that customers will ultimately see more innovation from Lonza.
“We are focused on developing the right organization and culture,” said Kicklighter. “We certainly have a great talent pool.”
The acquisition also expands Lonza’s global footprint. Prior to the purchase, Lonza was historically strong in North America, Europe and Asia. Arch extends the Lonza footprint in these regions, while creating a bigger presence in South America, Africa and across the Asia-Pacific region.
Moreover, the acquisition of Arch brought into the Lonza fold an additional 17 production and R&D facilities, including the Innovation & Technology Center in Alpharetta, GA, which Kicklighter called a “world-class” facility. Opened in September 2011, the $10 million, over 65,000 square-foot facility includes space dedicated to innovation and process technology, applications research, GLP analytical labs, and a top-of-line microbiology infrastructure. For its customers, the Arch acquisition immediately creates a broader array of product offerings and services, but Kicklighter insisted that household, personal product and I&I marketers will reap many more benefits.
“We have a strong commitment to innovation,” assured Kicklighter. “The new business will increase research and development and new product development across all platforms.”
With that kind of commitment, household and personal product marketers are sure to benefit from Lonza’s acquisition of Arch.
TAKEN FROM HAPPI MAGAZINE JAN 2012
Vistas de página en total
GREEN CHEMICALS
Also the materials have to meet with toxicity and health requirements regarding inhalation, dermal and eye contact. There is also a specific list of materials that are prohibited or restricted from formulations, like ozone-depleting compounds and alkylphenol ethoxylates amongst others. Please go to http://www.greenseal.com/ for complete information on their requirements.
For information on current issues regarding green chemicals, see the blog from the Journalist Doris De Guzman, in the ICIS at: http://www.icis.com/blogs/green-chemicals/.
Certification is an important — and confusing — aspect of green cleaning. Third-party certification is available for products that meet standards set by Green Seal, EcoLogo, Energy Star, the Carpet & Rug Institute and others.
Manufacturers can also hire independent labs to determine whether a product is environmentally preferable and then place the manufacturer’s own eco-logo on the product; this is called self-certification. Finally, some manufacturers label a product with words like “sustainable,” “green,” or “earth friendly” without any third-party verification.
“The fact that there is not a single authoritative standard to go by adds to the confusion,” says Steven L. Mack M.Ed., director of buildings and grounds service for Ohio University, Athens, Ohio.
In www.happi.com of June 2008 edition, there is a report of Natural formulating markets that also emphasises the fact that registration of "green formulas" is very confused at present, due to lack of direction and unification of criteria and that some governmental instittion (in my opinion the EPA) should take part in this very important issue.