Oil pollution, particularly of navigable waters, has excited more public concern than any other waste or spill material. This oil pollution has steadily increased with the increased oil consumption.
Crude oil released to the marine environment through accidental spillage or drainage from land causes serious damage to the environment and marine life .It is subjected to a wide variety of weathering processes which include evaporation, dissolution, dispersion, photochemical oxidation, microbial degradation, adsorption onto suspended material, agglomeration, etc.(1). These physicochemical changes in the oil enhance its dissolution in sea water.
Previous studies showed that photo oxidation of oil in the aquatic environment leads to the formation of numerous oxygenated products such as aromatic, aliphatic, benzoic and naphthanoic acids, alcohols, phenols and aliphatic ketones(2). Some of these products like aromatic and aliphatic acids, medium-molecular weight aromatic alcohols and ethers are water soluble. The most toxic effect is caused by water soluble aromatic derivatives that freshly spilled crude oil contains.
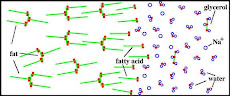
The methods that are commonly used to remove oil, involve booms, skimmers, sorbents etc .The main limitations of some of these techniques are their high cost and inefficient trace level adsorption(3). Surfactants are the active (i.e., interfacial tension reducing) ingredients in dispersants. Surfactants are compounds that have hydrophobic and hydrophilic components within the same molecule. The amphiphilic character of surfactants causes them to accumulate at interfaces because the hydrophilic part of the molecule interacts strongly with water, but the hydrophobic part is displaced from the aqueous phase because it interferes with more thermodynamically-favorable hydrogen bonding interactions between water molecules(4). When applied to a film of oil, the surfactants diffuse to the oil/water interface. There, they align themselves so that the lipophilic end of the molecule is attached to the oil phase and the hydrophilic end extends into the water phase. This reduces the interfacial surface tension between water and oil.
Nonionic surfactants are most common in dispersants because they are more effective, are generally less toxic, have a much lower aqueous solubility, and are less affected by electrolyte concentration than are anionic and cationic surfactants(5) . The nonionic surfactants used in commercial surfactants are ethoxylated derivatives of fatty acids, fatty alcohols, and fatty acid esters of sorbitan(6-8) .
However, the use of dispersant surfactants is the most widely employed and immediately effective method of combating oil pollution both on sea and on beaches. Surfactants applied to oil slick would cause the slick to break up into tiny droplets which would disperse throughout the water column. This effect causes the surfactants to accelerate natural oil weathering processes, and make the slick more amenable to biodegradation by increasing the available surface area. For these reasons the use of dispersants has become quite wide -spread worldwide (9-11).
The behavior of surfactant is strongly affected by the balance between the hydrophilic and lipophilic groups in the molecule (HLB) (12).
This work aims at preparing some nonionic dispersants and evaluating their effectiveness in dispersing and removing oil spills from contaminated water samples
Taken from a colleague collaborator in the petroleum and gas journal
viernes, 21 de enero de 2011
OIL SPILLS AT SEA AND THE ROLE OF DISPERSANTS
Suscribirse a:
Enviar comentarios (Atom)
Vistas de página en total
GREEN CHEMICALS
The Green Seal certification is granted by the organization with that name and has a great number of members contributing with the requirements to pass a raw material or a chemical product as "green". Generally for a material to be green, has to comply with a series of characteristics like: near neutral pH, low volatility, non combustible, non toxic to aquatic life, be biodegradable as measured by oxygen demand in accordance with the OECD definition.
Also the materials have to meet with toxicity and health requirements regarding inhalation, dermal and eye contact. There is also a specific list of materials that are prohibited or restricted from formulations, like ozone-depleting compounds and alkylphenol ethoxylates amongst others. Please go to http://www.greenseal.com/ for complete information on their requirements.
For information on current issues regarding green chemicals, see the blog from the Journalist Doris De Guzman, in the ICIS at: http://www.icis.com/blogs/green-chemicals/.
Certification is an important — and confusing — aspect of green cleaning. Third-party certification is available for products that meet standards set by Green Seal, EcoLogo, Energy Star, the Carpet & Rug Institute and others.
Manufacturers can also hire independent labs to determine whether a product is environmentally preferable and then place the manufacturer’s own eco-logo on the product; this is called self-certification. Finally, some manufacturers label a product with words like “sustainable,” “green,” or “earth friendly” without any third-party verification.
“The fact that there is not a single authoritative standard to go by adds to the confusion,” says Steven L. Mack M.Ed., director of buildings and grounds service for Ohio University, Athens, Ohio.
In www.happi.com of June 2008 edition, there is a report of Natural formulating markets that also emphasises the fact that registration of "green formulas" is very confused at present, due to lack of direction and unification of criteria and that some governmental instittion (in my opinion the EPA) should take part in this very important issue.
Also the materials have to meet with toxicity and health requirements regarding inhalation, dermal and eye contact. There is also a specific list of materials that are prohibited or restricted from formulations, like ozone-depleting compounds and alkylphenol ethoxylates amongst others. Please go to http://www.greenseal.com/ for complete information on their requirements.
For information on current issues regarding green chemicals, see the blog from the Journalist Doris De Guzman, in the ICIS at: http://www.icis.com/blogs/green-chemicals/.
Certification is an important — and confusing — aspect of green cleaning. Third-party certification is available for products that meet standards set by Green Seal, EcoLogo, Energy Star, the Carpet & Rug Institute and others.
Manufacturers can also hire independent labs to determine whether a product is environmentally preferable and then place the manufacturer’s own eco-logo on the product; this is called self-certification. Finally, some manufacturers label a product with words like “sustainable,” “green,” or “earth friendly” without any third-party verification.
“The fact that there is not a single authoritative standard to go by adds to the confusion,” says Steven L. Mack M.Ed., director of buildings and grounds service for Ohio University, Athens, Ohio.
In www.happi.com of June 2008 edition, there is a report of Natural formulating markets that also emphasises the fact that registration of "green formulas" is very confused at present, due to lack of direction and unification of criteria and that some governmental instittion (in my opinion the EPA) should take part in this very important issue.















































No hay comentarios:
Publicar un comentario